
The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. The cookie is used to store the user consent for the cookies in the category "Performance". This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. The cookies is used to store the user consent for the cookies in the category "Necessary". The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". The cookie is used to store the user consent for the cookies in the category "Analytics". These cookies ensure basic functionalities and security features of the website, anonymously. Necessary cookies are absolutely essential for the website to function properly. When he did this he noted that the chemical properties of the elements and their compounds showed a periodic trend. Mendeleev arranged the elements in order of increasing relative atomic mass. What properties did Mendeleev use to arrange the elements? The Mendeleev periodic table is based on the periodic law, that elements are arranged in the order of increasing their atomic weights. What is the basis of Mendeleev periodic law? Mendeleev’s periodic table made it possible to predict properties of elements that had not yet been discovered. His organization of elements was based on atomic mass. Mendeleev published his periodic table in 1869. What is the importance of Mendeleev’s periodic law? Because the properties repeated themselves regularly, or periodically, on his chart, the system became known as the periodic table. When Mendeleev arranged the elements in order of increasing atomic mass, the properties where repeated. He found that every eight elements had similar properties and called this the law of octaves. These atomic weights should be considered provisional since a new isotope with a longer half-life could be produced in the future.Who first arranged the elements according to mass?īritish chemist John Newlands was the first to arrange the elements into a periodic table with increasing order of atomic masses. The convention is to list the atomic mass of the longest-lived isotope in the periodic table. For lab-created trans-uranium elements - elements with atomic numbers higher than 92 - there is no "natural" abundance.

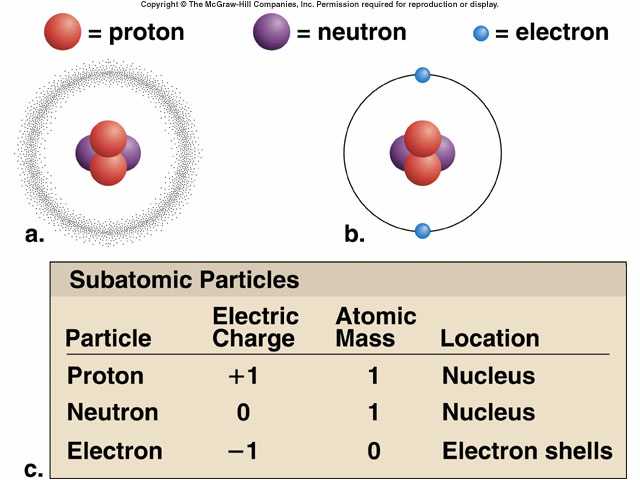
The average number of neutrons for an element can be found by subtracting the number of protons (atomic number) from the atomic mass. Individual atoms always have an integer number of atomic mass units however, the atomic mass on the periodic table is stated as a decimal number because it is an average of the various isotopes of an element.

For example, carbon atoms have six protons, hydrogen atoms have one, and oxygen atoms have eight.Ītomic mass - For naturally occurring elements, the standard atomic mass of an element is the average mass of the element in atomic mass units (amu). The number of protons defines what element it is and also determines the chemical behavior of the element. Also, the atomic symbol for gold if "Au" because the word for gold in Latin is aurum.Ītomic number - The number of protons in an atom is referred to as the atomic number of that element. For example, the symbol for tungsten is "W" because another name for that element is wolfram. These symbols are used internationally and are sometimes unexpected. The number of electrons in a period increases as one moves down the periodic table therefore, as the energy level of the atom increases, the number of energy sub-levels per energy level increases.Įlements that occupy the same column on the periodic table (called a " group") have identical valance electron configurations and consequently behave in a similar fashion chemically.įor each element, the Periodic Table indicates very important informations:Ītomic symbol - The atomic symbol (or element symbol) is an abbreviation chosen to represent an element ("C" for carbon, "H" for hydrogen and "O" for oxygen, etc.). The period number of an element signifies the highest energy level an electron in that element occupies (in the unexcited state), according to the Los Alamos National Laboratory.

Order generally coincides with increasing atomic mass.
Meyer accounted for two important features that are usually attributed only to Mendeleev: he reversed the order of tellurium and iodine, and he left gaps. Elements are arranged from left to right and top to bottom in order of increasing atomic number. In his table, Meyer organized the elements according to their atomic masses and valences, the latter of which had been discovered in the 1850s. The Periodic Table of Chemical Elements arranges all of the known chemical elements in an informative array.


 0 kommentar(er)
0 kommentar(er)
